The last post “Silicon Anodes: Big Promises Not Yet Delivered” on January 7th discussed silicon anodes for electric car batteries. There has been quite a bit of talk of silicon anodes and it does appear the California-based company featured in the post, Nanograf, is on the cusp of bringing its silicon-graphite hybrid anode material to market. However, a true silicon-based anode appears to need much more development.
If and when,
affordable silicon anodes appear in the market, it could be a significantly
disruptive technology, shifting demand for metals and the fortunes of several
industries in the upstream supply chain for batteries. Metals mining companies, materials processors
and anode manufacturers would all be impacted. It makes sense for investors to watch these
segments of the battery supply chain for investment opportunities and know the
competitive risks that could be lurking along the supply chain.
Indeed, there are
a number of potentially disruptive battery technologies. This post looks are several battery
technologies that, along with silicon, could bring meaningful change to the
world of batteries.
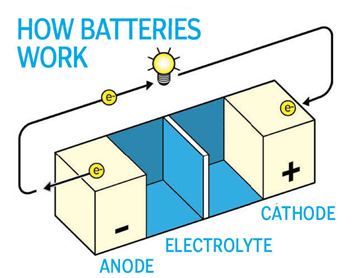
Magnesium - Lithium ion battery chemistry looks complicated with electrolyte navigating a ‘separator’ and making the charge/discharge round between the two metal anode and cathode ‘poles’. Magnesium has been tried as a solid anode and as the active charge in the electrolyte. With 50% higher volumetric energy density than lithium it is attractive as a replacement. Additionally, magnesium does not form those annoying tree-like structures called dendrites that lithium creates inside batteries. Thus magnesium can eliminate the need for additional dendrite-fighting compounds. Rid of that layer the magnesium battery cell has nearly 5 times the volumetric energy density of a lithium ion cell, potentially give the magnesium battery a cost advantage.
Room Temperature
Sodium Sulfur
- While not necessarily
interesting for electric car batteries, large scale grid energy storage could
benefit from batteries using improved sodium-sulfur chemistries. In the
conventional NaS battery, molten sodium is the negative electrode and molten
sulfur is the positive electrode. Safety
concerns over the high heat range over 300 degrees Centigrade have held back
their widespread adoption. The
temperature can be lowered to room ambiance with the use of a ‘cocktail’
electrolyte containing propylene and fluoroethylene carbonates as co-solvents,
and then lacing the mix with concentrated sodium salts with a pinch of indium
triiodide. The extra mixing makes
possible the high capacity and long cycling stability of NaS at a more
energy-efficient, safer temperature.
Graphite Dual-ion - Large-scale battery storage situations are also the target of graphite dual-ion batteries. The design relies on lithium salts for the electrolyte but uses graphite for both the cathode and the anode. During discharge, both anions and lithium ion return to the electrolye. The design makes it possible to fully discharge the battery without risking a short-circuit situation. It also operates at ambient temperatures, reducing the risk of thermal runaway and eliminating the need for the elaborate cooling systems that space and cost money.
Proton - As much a fuel cell as a battery, the proton battery is more or less a reversible hydrogen fuel cell. The design produces protons by splitting water and then stores the protons in a graphite electrode. The protons are then released again and pass back through the reversible fuel cell to form water using ambient air. Thus far in the development of proton batteries, energy per unit mass is on par with commercially-available lithium ion batteries. Developers are targeting the medium-scale electricity grids as well as commercial and residential applications.
Paper Polymer - Wish you could fit a long-lasting battery in your phone? Paper polymer batteries could be the answer. Thinner than any battery on the market today, a paper polymer battery features a cellulosic spacer and nanoscale structures that serve as high-surface area electrodes. In addition to low cost of production and high energy performance benefits, paper polymer batteries can claim operational safety and environmental friendliness as benefits.
Aluminum Ion - Move over lithium ions! Aluminum ions can be used in the battery electrolyte too, flowing from the positive battery electrode or anode, to the negative electrode or cathode. Aluminum ions can exchange three electrons per ion, making each one equivalent to three lithium ions in a conventional battery. Developers are working to improve host materials for stable electrochemical behavior in the face of such strong energy capacity. It is worth the effort since aluminum is readily available in a number of locations around the world and is relatively easy to handle at ambient temperatures. Furthermore, the form factor for an aluminum ion battery is potentially smaller than a lithium ion counterpart creating savings for manufacturers.
Potassium Ion - Another analogue of the lithium ion battery is a design that uses potassium ions to transfer the charge instead of lithium ions. Graphite anodes can be used with the potassium-laced electrolyte, but low capacity retention is a problem that must be overcome. Carbon nanotubes and nanofibers have been tried as well as phosphorous-doped carbon materials. A variety of potassium metal oxide materials have been tried as the cathode material. Electrolytes for this battery also require an upgrade from the conventional lithium ion battery deal with safety concerns.
Salt Water - There is so much focus on the battery anode
and cathode, the electrolyte often goes unnoticed. The electrolyte is the liquid medium that
carries the ion transporter between the cathode and anode. In the salt water battery, this important
battery component is a concentrated saline solution. The sodium works as the energy
transporter. Salt water batteries have
several characteristics that give this battery design strong commercial value. This design avoids harmful materials that are
required in conventional lithium ion batteries, making salt water batteries
environmentally friendly.
Often small companies
- our favored sector for investment
- are the champions of new
technology. In the next post we look at
a few companies pursuing these technologies and that could be good investment
targets.
Neither the author of the Small Cap Strategist web
log, Crystal Equity Research nor its affiliates have a beneficial interest in
the companies mentioned herein.
No comments:
Post a Comment