PRIME SERIES
References to abuse and dependence on alcohol trace
back to the earliest Egyptian and Babylonian writings. Yet the idea that excessive alcohol use causes
problems had mixed acceptance. The
adverse effects of habitual alcohol abuse did not gain widespread recognition
until the mid-19th century when ‘alcoholism’ finally appeared in the
lexicon.
It has not been until the last few decades that any
significant progress has been made in understanding the effects of alcohol on
the human body. It warrants a new name -
alcohol use disorder (AUD) - to fully encompass the many paths to
dependence on alcohol and the understanding of it as a chronic relapsing brain disease.
Even as
scientists have gained insight into alcohol’s ravages on mind and body, effective
treatments for alcohol use disorder (AUD) have remained limited. Early stage biotech Adial Pharmaceuticals, Inc. (ADIL: Nasdaq) is trying to
change that with development of a therapeutic compound based on ondansetron, a
well known selective antagonist of the serotonin. Adial
is building on earlier work using ondansetron as a treatment of severe nausea
and vomiting. The company is about to
begin a clinical trial targeting binge drinkers
- the only Phase III clinical
trial in the world set to begin in 2019 - that could bring relief to a
disorder that causes pain and loss to millions.
An Underserved Market
According to the
National Survey on Drug Use and Health (NSDUH) completed in 2015, an estimated
15.1 million people in the United States over the age of 18 years suffered from AUD. The survey found the group is tilted toward men - 9.8 million men versus 5.3 million women.
Adial management believes the situation is even more dire than suggested by the NSDUH. They point to the National Epidemiologic Survey on Alcohol Related Conditions (NESARC) published in the Journal of the American Medical Association (JAMA) in 2015, that used a more recent, broader definition of the disease. This survey found 30 million people over the age of 18 years had AUD in 2013. For perspective that was 13.9% of the U.S. population in 2015 - 17.6% of adult men and 10.4% of adult women.
Tragically, at the time those surveys were completed, only about 7% of the adults who were thought to have AUD received treatment.
Adial management believes the situation is even more dire than suggested by the NSDUH. They point to the National Epidemiologic Survey on Alcohol Related Conditions (NESARC) published in the Journal of the American Medical Association (JAMA) in 2015, that used a more recent, broader definition of the disease. This survey found 30 million people over the age of 18 years had AUD in 2013. For perspective that was 13.9% of the U.S. population in 2015 - 17.6% of adult men and 10.4% of adult women.
Tragically, at the time those surveys were completed, only about 7% of the adults who were thought to have AUD received treatment.
With so many
people going without help, it is no surprise that the disease is costly. The associated social and health effects of
AUD are serious - accidents, violence, domestic abuse, chronic
heart and liver disease, fetal alcohol syndrome, loss of employment. The U.S.
Centers for Disease Control and Prevention (CDC) estimates the economic cost of
alcohol abuse and dependence is as much as $249 billion each year in the U.S. The CDC researchers determined the costs are
primarily associated with loss in workplace productivity from absenteeism as
well as workers’ compensation, unemployment and disability payments.
What is even more
compelling for developers like Adial is that those that do seek treatment are
often limited to detoxification and abstinence programs that may not work. A 2013 study found that about 20% of those
receiving treatment for AUD returned to pre-treatment levels of alcohol use within a year. Success rates for well-known
twelve-step programs may be as low as 5%, while the American Society of Addiction Medicine (ASAM) pegs the Alcoholism Anonymous success rate at 10%.
Limited Options, Large Obstacles
There are AUD treatment
options that have been on the market for some years. Beside motivational enhancement therapy and
cognitive-behavioral treatment there are three prescription medications
approved for use in the U.S. - disulfiram, naltrexone and acamprosate. Nalmefene has also been approved in Europe. The main goals of these treatments are to
either help patients avoid alcohol entirely or to reduce alcohol use, both of
which have been shown to deliver improvement in health, safety and
quality of life. Yet, all four are dependent upon
full abstinence or an ongoing detoxification effort.
ALCOHOL ABUSE MEDICATIONS
|
||||
Compound
|
Brand Name
|
Developer/Producer
|
Action
|
Side Effects
|
Disulfiram
|
Antabuse
|
Teva Pharmaceuticals
|
Inhibits acetaldehyde dehydogenase enzyme in liver that breaks down
alcohol
|
Violent reactions to alcohol,
confusion, seizures
|
Naltrexone
|
Vivitrol, Depade, ReVia
|
Alkermes, Plc.
Mallinckrodt Pharma.
Teva Pharmaceuticals
|
Opioid-blocking drug operating through risk-reward feedback in brain
|
Stomach cramping, anxiety, headache, nausea, sleep loss
|
Acamprosate
|
Campral
|
Allergan, Inc. (Forest)
Mylan Pharmaceuticals
|
Mimics neurotransmitters, stabilizes signaling during alcohol
withdrawal
|
Diarrhea, nausea, loss of appetite, vision problems, memory loss
|
Nalmefene
|
Selincro
|
H. Lundbeck AS
|
Prevents alcohol-induced release of dopamine
|
Nausea, headache, dizziness, insomnia
|
Source: Corporate presentations
|
||||
Unfortunately, these approved treatments are often contraindicated by a patient’s unique physiology. What is more the sometimes brutal side effects of these drugs can give pause even to the most desperate AUD suffer. Disulfiram causes such violent reactions to alcohol it not only serves as a deterrent to consumption of even the smallest toddy, it can lead to vision changes, extreme confusion and seizures. All four drugs have reputations for stomach pain and nausea.
As a consequence
only about 6% of people who say they have AUD are prescribed any medication to
help with stopping and avoiding alcohol abuse.
This leave wide open an opportunity to serve AUD suffers with an
effective and tolerable therapeutic alternative.
There is
building knowledge of the brain and how alcohol affects it, making possible
improvements on these therapies. One of
the revelations is that genetic influences may predispose certain individuals
to develop AUD. Adial’s Chairman, Dr.
Bankole Johnson, has been one of the leading researchers in capturing the
genetic profile of AUD and paving the way to more effective therapies.
New Research Focus
Adial’s lead
investigational compound, AD04, is a
selective serotonin-3 antagonist that the company wants to target at certain
patients with a particular genetic make-up.
Serotonin is a neurotransmitter in the human body that is believed to
help regulate mood, social behavior and the risk-reward mechanism. The serotonin antagonist is believed to
interrupt the reinforcing effects of alcohol in the brain that drives the urge
to drink.
The active
ingredient in AD04 is ondansetron,
the first serotonin-3 antagonist to receive FDA approval. Its developer, GlaxoSmith Kline (GSK:
NYSE) first established efficiency in the late
1980s in animal models and continued with extensive studies that resulted in a
treatment for severe nausea and vomiting that is now marketed as Zofran.
Adial has an
exclusive license to AD04 from the
University of Virginia Patent Foundation, which holds three separate patents on
the technology. Under the direction of Dr. Johnson, then an addiction
researcher and chairman of the University of Virginia (UVA) Department of Psychiatry,
a Phase 2b clinical trial was completed involving 238 patients with AUD that
were given ondansetron. Adial has
access to all the data from this early trial that showed a statistically
significant difference between ondansetron and placebo doses. The endpoints of the trial were reduction in
severity of drinking as measured by drinks per day and reduction in the
frequency of drinking as measured in days of abstinence.
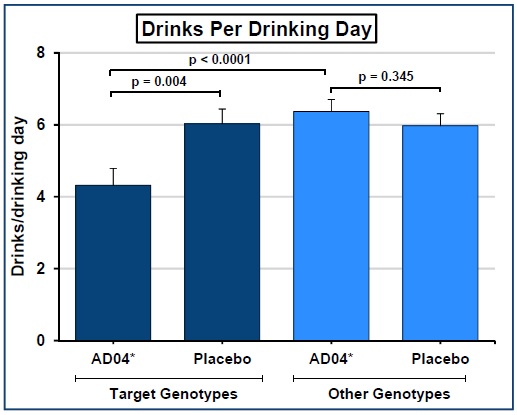 |
| AD04 Trial Results Summary |
Adial also
benefits from the original safety clinical trials for ondansetron. Studies of the compound leading up to FDA
approval of Zofran for nausea, involved does as to almost 100 times the dosage
anticipated for AD04. Even at high doses ondansetron was
well-tolerated and resulted in few adverse effects. However, in the Phase 2B trial at UVA, participants
were actually given low doses of ondansetron.
Phase 3 Clinical Trial
 |
| Serotonin Receptor Structure |
Ultimately, the
biomarker test will be a requirement posted on its label before prescribing an approved AD04 compound. Adial will seek regulatory approval of the
test, simultaneously with approval of the AD04
compound.
Opening Door to Change in AUD Diagnosis and Treatment
The biomarker
test, which is expected to be low-cost, fast and accurate, could be used by
physicians as pre-treatment screening step.
The marriage of the test with the treatment may increase the likelihood
of success for the patient who could receive a treatment most suited to his or
her genetic make-up and resulting pathway to alcohol abuse.
Although Adial
management believes psychiatrists and addiction specialists will be among the
first to begin using AD04, the
biomarker test and then the drug could be administered by primary care physicians as well. Frontline medical professionals often see
patients who exhibit signs of problem drinking but are resistant to a
conversation about AUD. The biomarker
test could be an opening to an effective care program that might not otherwise
be undertaken. Use of an objective test is expected to remove some of the
social stigma associated with the disease that may discourage patients who fear
the label ‘alcoholic.’
The linkage of
the biomarker test as a pre-treatment screen in the physician’s office may
represent fundamental change in AUD treatment.
The change in the exam room could broadly expand number of people who
seek AUD treatment.
Ahead of the Pack
There are other
compounds under investigation for use in treating AUD, but none appear poised
to have a significant impact on the AUD treatment.
Baclofen, a
selective agonist for the GABA-B receptor, is sold under the name Lioresal and
other generic names for the treatment of muscle spasm. Three large clinical trials for baclofen
involving over 600 patients with AUD were completed in 2016, but produced mixed
results. Only one of the three found any
difference in alcohol consumption and abstention between baclofen and placebo
groups.
Gabapentin, a
calcium channel blocker, also impacts GABA-B production. Xenoport, Inc., now owned by Arbor
Pharmaceuticals LLC (privately held), orchestrated a clinical trial in 2014,
involving 150 alcohol-dependent participants found that gabapentin was
effective in treating relapse symptoms.
Financing Secured
Adial management
estimates it will require an investment of approximately $6.5 million to complete the initial
Phase 3 clinical trial for AD04. Approximately, $2.5 million of capital raise
in the company’s initial public offering in July 2018, has been earmarked for
the trial budget. Management believes
its work will qualify for existing grant programs, which could be used as part
of the other $4.0 million in required to complete the initial Phase 3 trial.
A second, larger
Phase 3 trial is planned with as many as 580 participants. The company will set up the second trial with
the same screening approach as in the first trial. However, the desired end point will be simpler
- no heavy drinking days in last two
months of the trial. The larger trial
will require additional capital, but by then Adial management expects to have
data from the first trial to help gain interest from investors.
Valuing Sober Patients
Adial management
is confident on future valuation for its contribution to AUD treatment. The team looks to the successful trajectory
of the buprenorphine and naloxone compound developed and marketed as Suboxone
by Reckitt Benckizer Pharmaceuticals and now Indivior, Plc (INDV: L). Suboxone has been called the ‘gold standard’
of medication-assisted treatment of opioid dependence. Before patent protection ended and generic alternatives
came on the market, Suboxone accounted for 85% of spending on
medicated-assisted opioid dependence treatment.
The Adial team, which includes as Adial director and former Indivior business development officer Tony Goodman, aspires to duplicate the Suboxone market success. Adial hopes to earn
similar respect from biotech investors that tripled Indivior shares within three
years of its IPO. Even after a recent
sell-off the Indivior addiction dependence play still gives early investors a
72% gain since the going public action in 2015.
Part II of this two part series on Adial
Pharmaceutical will look at the company’s financial profile and its promising
technology to treat alcohol abuse disorder.
PUBLIC COMPANIES MENTIONED IN THIS
ARTICLE
|
||||||
Company
|
SYM
|
Price
|
Mkt Cap
|
Sales
|
EPS
|
PE
|
Teva Pharmaceuticals
|
TEVA
|
$22.85
|
$23,270
|
$20,780
|
-$10.09
|
Neg
|
Alkermes, Plc
|
ALKS
|
$39.66
|
$6,160
|
$1,020
|
-$0.92
|
Neg
|
Mallinckrodt Pharmaceutical
|
MNK
|
$30.50
|
$2,540
|
$3,270
|
$18.59
|
1.64
|
Allergan, Inc.
|
AGN
|
$188.22
|
$63,890
|
$16,160
|
-$5.32
|
Neg
|
Mylan Pharmaceuticals
|
MYL
|
$37.48
|
$19,320
|
$11,720
|
$0.87
|
43.23
|
H. Lundbeck AS
|
LUN.CO
|
$60.65
|
$1,903
|
$2,847
|
$2.89
|
21.01
|
GlaxoSmithKline
|
GSK
|
$39.01
|
$94,880
|
$39,680
|
$0.88
|
44.13
|
Indivior Plc
|
INDV.L
|
$352.49
|
$2,561
|
$1,407
|
$12.34
|
28.56
|
Pfizer Pharmaceuticals
|
PFE
|
$42.96
|
$251,840
|
$53,240
|
$3.74
|
11.47
|
Adial Pharmaceutical, Inc.
|
ADIL
|
$2.75
|
$18
|
$0
|
-$0.41
|
Neg
|
*All figures in USD; Market cap and
sales in millions
|
||||||
Neither the author of the Small Cap Strategist web
log, Crystal Equity Research nor its affiliates have a beneficial interest in
the companies mentioned herein.
Underwriters of the Prime series may have a beneficial
interest in, serve as agents of, or act as advisers to the companies mentioned
herein.
No comments:
Post a Comment