PRIME SERIES
Clearmind Medicine
(CMND) is a clinical stage pharmaceutical company with the promising CMND-100
candidate at the front end of its pipeline. CMND-100 is based on the psychoactive compound
MEAI, shorthand for the synthetic molecule 5-MEthoxy-2-AminoIndine. The Company purchased the compound in 2021,
and has since completed several pre-clinical studies in animals to establish safety
and metabolic profiles.
One of the pre-clinical
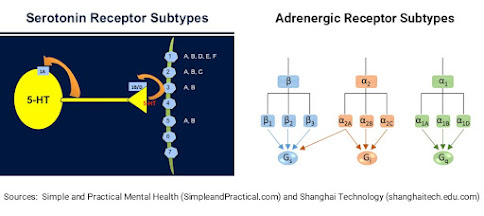
studies tested MEAI as a curb for alcohol cravings in ‘binge drinking’ mice. The MEAI compound interacts with central nervous
system elements, specifically serotonergic receptors 5-HT1A and 5-HT2B as well
as adrenergic receptors α2A, α2B and α2C. The receptor 5-HT1A is particularly important
in alcohol dependence, so agonists of this receptor could help in reducing
alcohol intake. Importantly, MEAI may
have application for other binge-like diseases such as cocaine abuse and overeating.
Results for
early tests have been promising, prompting the Company to make plans for human
clinical trials. In early 2023, an Investigational
New Drug (IND) application was submitted to the U.S. Federal Drug
Administration (FDA) for the first human clinical trials of MEAI as CMND-100
aimed at patients with Alcohol Abuse Disorder or AUD.
The U.S. trials will be conducted at the Yale School of Medicine’s Department of Psychiatry and Johns Hopkins University School of Medicine. A similar application has been submitted to Israel’s Pharmaceutical Division of the Ministry of Health, which regulates the drug registration process in that country.
Speculative
and Compelling
Shares of Clearmind
Medicine offer a stake in a promising compound aimed at diseases with
increasing incidence and few effective treatments. The bull case for the stock balances the
risks of an early-stage company with the opportunity of large, but underserved patient
groups.
In this author’s
view, the stock is best suited for investors with a palate for the unique risks
inherent in pharmaceutical development
- an average 10% probability for
successful completion of clinical trials and regulatory approval of newly
discovered therapeutic compounds. Yet,
once the details are hammered out, regulatory approval of CMND-100 could
open the door to a market primed for effective therapies of binge behavior. As is discussed below, sales and profit
potential in the AUD market alone is compelling.
Taking psychoactive
therapies seriously
Mention psychedelic
drugs and many people might think first of LSD and the drug fueled 1960’s and
1970’s -
not a therapy to be taken seriously by the medical community. It is time to recalibrate! Psychoactive compounds may have effective
properties for the treatment of numerous medical conditions.
Indeed, the U.S.
Federal Drug Administration has taken note and is ready to entertain regulatory
petitions for potential drug products.
In August 2023, the FDA issued draft guidance for drug developers
entitled “Psychedelic
Drugs: Considerations for Clinical
Investigations – Guidance for Industry”.
The FDA followed this publication with a virtual public workshop
entitled “Advancing
Psychedelic Clinical Study Design” for researchers and industry.
Perhaps Clearmind
scientists could have benefited from the new guidance if the FDA had published
it a few months earlier. Nonetheless, in
January 2024, a Type A meeting with Clearmind scientists and FDA
representatives has apparently tightened up and refined the Company’s IND application
to proceed with tests of its CMND-100 in humans. In recent conversations with this author, Clearmind
management expressed confidence in winning the FDA go-ahead for a Phase I clinical
trial in 2024.
Lucrative
target market for profit….or critical community service
The poignant difficulties
experienced by AUD suffers in terms of disruption in relationships, loss of
employment and costs of illness might be enough to attract some scientists to work
on the problem. However, for
profit-oriented companies and their investors, the market presents a particularly
lucrative investment opportunity.
Alcohol abuse is
among the most frustrating and costly diseases suffered by humans. The National
Center for Drug Abuse Statistics (NCDAS) estimates AUD along with alcoholism
kills over three million people each year, representing 6% of global deaths. Additionally, the financial burden of alcohol
misuse is enormous. Indeed, the UNC
School of Medicine Bowles Center for Alcohol Studies estimates over 15% of
the entire national health care budget in the U.S. is spent on treating
conditions related to substance abuse, including alcohol. The Centers
for Disease Control and Prevention takes a view from a higher vantage
point, estimating excessive alcohol use results in an economic loss of $249
billion annually from the U.S. economy alone.
With an economic
significance of such import, it follows that the market opportunity for AUD solutions
is large and growing. Future
Market Insights, an industry research firm, estimates the global AUD
treatment market generated $700 million in value in 2023. Within the next ten years the firm estimates
the rising incidence of AUD and binge drinking could drive the market value to
$1.3 billion by 2033.
Competitors
and Peers
Presently there
are a handful of medical treatments for AUD.
Despite long-time approval by the U.S. FDA, none have captured dominant market
share. The segment may have also lost a participant.
Sanofi announced
in April 2023, it would be taking its Antabuse (disulfiram) off the market citing
supply chain issues. However, thin margins
on a low-priced product probably also played a role. Disulfiram stops the body from breaking down
ethanol in alcoholic beverages, leading to such severe headaches, nausea and
vomiting. The effects are so severe patients
are dissuaded from consuming alcohol. The
fact that many patients are disappointed in Sanofi’s decision reveals the lengths
to which AUD suffers will go to get relief.
Notably, two companies
have naltrexone-based therapies on the market.
Originally intended for emergency treatment of opioid overdose, naltrexone
has its own side effects, such as stomach cramping, anxiety, headache and trouble
sleeping. Naltrexone packaging also carries
the hated FDA black box with a warning for potential liver damage if taken at
doses higher than recommended. Despite
the side effects, naltrexone branded as Vivitrol in 2023 earned an estimated $410
million in annual sales for its owner Alkermes plc (ALKS).
|
Regulatory
Approved Therapies for AUD |
|||
|
Compound Name |
Brand Name |
FDA Approval |
Manufacturers |
|
Disulfiram |
Antabuse |
1949 |
Sanofi (SNY: NYSE) |
|
Naltrexone |
ReVia |
1994 |
Teva Pharma (TEVA: NYSE) |
|
Acamprosate |
Campral |
2004 |
Forest Sub of Allergan (AGN: NYSE) |
|
Naltrexone |
Vivitrol |
2006 |
Alkermes, plc
(ALKS: NYSE) |
There are other
developmental stage companies vying for the next FDA approved AUD
treatment. For example, Adial Pharmaceuticals
(ADIL) is working on AD04, a serotonin-3 antagonist targeted at certain genetically
qualified patients suffering from AUD. Currently,
the company is planning two Phase III trials to satisfy regulatory applications
in both the U.S. and European Union.
Bootstrapping
to Higher Valuation
The aspirations
of Clearmind’s scientists are all just talk without adequate capital to complete
required development work and clinical trials.
In January 2024, the Company raised a total of $2.4 million in new capital
through the sale of 1.5 million shares of common stock and another 1.5 million warrants.
After the recent
capital raise capital resources are estimated near $6.05 million. (Cash at the end of July 2023: $3.85M + Exercise of warrants: $3.50M + Offering proceeds: $2.4M – Estimated cash used to support
operations through Jan. 2024: $3.70M) Management has guided for current cash reserves
closer to double digits, suggesting a significant reduction in cash usage in
the last six months while the Company has hammered out details for its IND application
to the U.S. FDA.
In a recent
conversation with this author, the Clearmind team expressed confidence in having
the resources to complete planned clinical trials for CMND-100. This would be an important achievement, given
that management would have data from Phase I clinical trials to cite if additional
capital is needed for the next step. The
Company can thus artfully accomplish what sometimes eludes developmental stage
companies: minimizing dilution by raising
only just enough capital to achieve measurable results and then using those
results to bootstrap to a higher valuation in the next financing effort.
Value
drivers…multiple treatment applications
This article has
focused on the AUD treatment application.
However, that might be selling the Company short on the potential in CMND-100.
The MEAI compound may have applications for
obesity and drug addiction as well.
Clearmind recently
cooperated with research scientists at the Bar-Ilan University in Israel in
conducting pre-clinical trials of the Company MEAI molecule in animals
conditioned with cocaine. The trial
results suggested MEAI could be helpful in reducing cravings and altering
behaviors. Importantly, the trials
established the MEAI compound is not itself addictive. Clearmind has a long-term license agreement
with BIRAD, the university’s research and development arm, to use a joint
patent for cocaine treatment.
Additionally,
Clearmind is collaborating with SciSparc Ltd. (SPRC), a clinical-stage
pharmaceutical company focused on disorders of the central nervous system. The SciSparc relationship is wide-reaching,
anticipating therapeutic applications for a lengthy list of mental health disorders
in addition to binge-like and addiction diseases.
Clearmind has
already filed six provisional patent applications with the U.S. Patent and
Trademark Office for combinations of psychoactive compounds using the Company’s
MEAI molecule alone as well as in combination with SciSparc’s proprietary
anti-inflamatory compound palmitoylethanolamide (PEA).
In November
2023, the partners announced results from a pre-clinical trial aimed at determining
optimal dosage of the MEAI-PEA combination.
The animals in the trial were observed for metabolic factors such as fat
oxidation, weight loss and reduced appetite.
The mice showed high tolerance at all dosage levels, reduction in food
consumption and improvement in fat oxidation.
Clearmind leadership thinks the study is encouraging for use of the MEAI
compound for obesity and metabolic disorders with the results supporting
further study with animals and eventually humans.
Clearmind has a growing
portfolio of patent protected intellectual property - 27
granted patents in the U.S., Europe and China with 24 patent applications
pending. Applications and awarded
patents cover both method of use and composition of matter, giving the Company
the means to prevent competitors from elbowing in with a competing MEAI
compound or from imitating the Company’s proprietary chemical mixtures.
Back of envelope
intrinsic value
It is possible
to value Clearmind’s entire patent portfolio, estimating sales and earnings
potential for each of the protected compounds.
To reduce the number of assumptions to a more manageable level, a valuation
exercise was undertaken based only at the Company’s lead candidate and primary
target application, CMND-100 as a treatment for AUD.
The preferred approach
for valuing pharmaceutical companies is the discounted cash flow method. Industry averages for an early-stage companies
were used for the critical assumptions.
In this calculation, the discount rate is used primarily to reflect the
time value of money. Additionally, estimated
cash flows were adjusted to reflect business perils, i.e. the possibility of
failure at the principal development stages.
(Key assumptions and data points are detailed below.)
The exercise
found a risk adjusted intrinsic value of $4.78 per share for Clearmind, almost four
times the current stock price. Aside
from the effects of one assumption or another in the final outcome of the valuation
exercise, the difference between market and estimated stock value is probably
large due to investors’ view on execution risk.
Much
potential, many possible stumbling blocks
And pre-clinical
trials have demonstrated its principal therapy CNMD-100 based on the psychoactive
compound MEIA shows promise as a safe treatment for binge drinking. Nonetheless, there is still plenty of
opportunity for the Company to stub its corporate toe.
Successful
pre-clinical trials do not guarantee smooth sailing in human clinical
trials. The Company has lined up research
partners at ‘blue ribbon’ universities, but even if execution is perfect, the
MEIA compound could fall short of expectations as the Phase I trials unfold.
Clearmind has established
at least two strong development partnerships, one of which is with SciSparc,
another public pharmaceutical developer with complementary intellectual
property. It is notable that Clearmind
Medicine’s chief executive officer, Dr. Adi Zuloff-shani, is also the lead
science officer at SciSparc Ltd. So far,
the dual role has created some efficiency for Clearmind as in her role at SciSparc
Zuloff-Shani has already completed some required steps in Clearmind’s
development agenda. For example, qualification
of manufacturers for active ingredients is common to both firms. However, in the future potential conflicts
could arise as the two companies more forward to more advanced development work.
The FDA’s recently
issued guidance for psychedelic compound development has also helped shine a
bright light on the therapeutic value of such compounds. Certainly, the FDA’s initiative is a plus for
CNMD shareholders, but there is still room for confusion or misstep. The FDA is still perfecting it position vis-à-vis
psychoactive compounds and could change its suggested plan of action to the
detriment of development projects already underway at Clearmind.
Conclusion
Besides new fortifying
financial resources, the Company’s recent capital raise helped elevate Clearmind’s
visibility in the pharmaceutical sector as a developer with the capacity to
deliver favorable early-stage clinical trial results. In the current year, the Company’s principal
compound CNMD-100 is likely to begin dosing and safety trials with
humans suffering from alcohol addiction.
Investors with an extended investment horizon and the tolerance for
significant risk may find this inflection point is a compelling opportunity for
a stake in a
·
potentially undervalued company
with a
·
promising compound CMND-100 aimed
at a
·
large and growing market for
alcohol dependence treatment.
Neither the author of the Small Cap
Strategist web log, Crystal Equity Research nor its affiliates have a
beneficial interest in the companies mentioned herein.
Underwriters of the Prime series may
have a beneficial interest in, serve as agents of, or act as advisors to the
companies mentioned herein.
Valuation
Assumptions
Commercial Stage: Model Year 6
AUD market value: current $700 million rising to $1.2 billion in Year 6
Market share: 10%
Cash earnings margin: 8%
Commercial stage growth rate: 10%
Cost of capital: 18%
Cash flow discount rate: 11%
Risk Adjustments: Phase I - 63%,
Phase II - 58%, Phase III - 85%
Commercial stage balance sheet: No
cash, no debt
Cash reinvestment in commercial stage: None
Current shares outstanding: 3.2
million
Risk adjusted estimated net present value: $15.29 million
Risk adjusted intrinsic value per share: $4.78
No comments:
Post a Comment